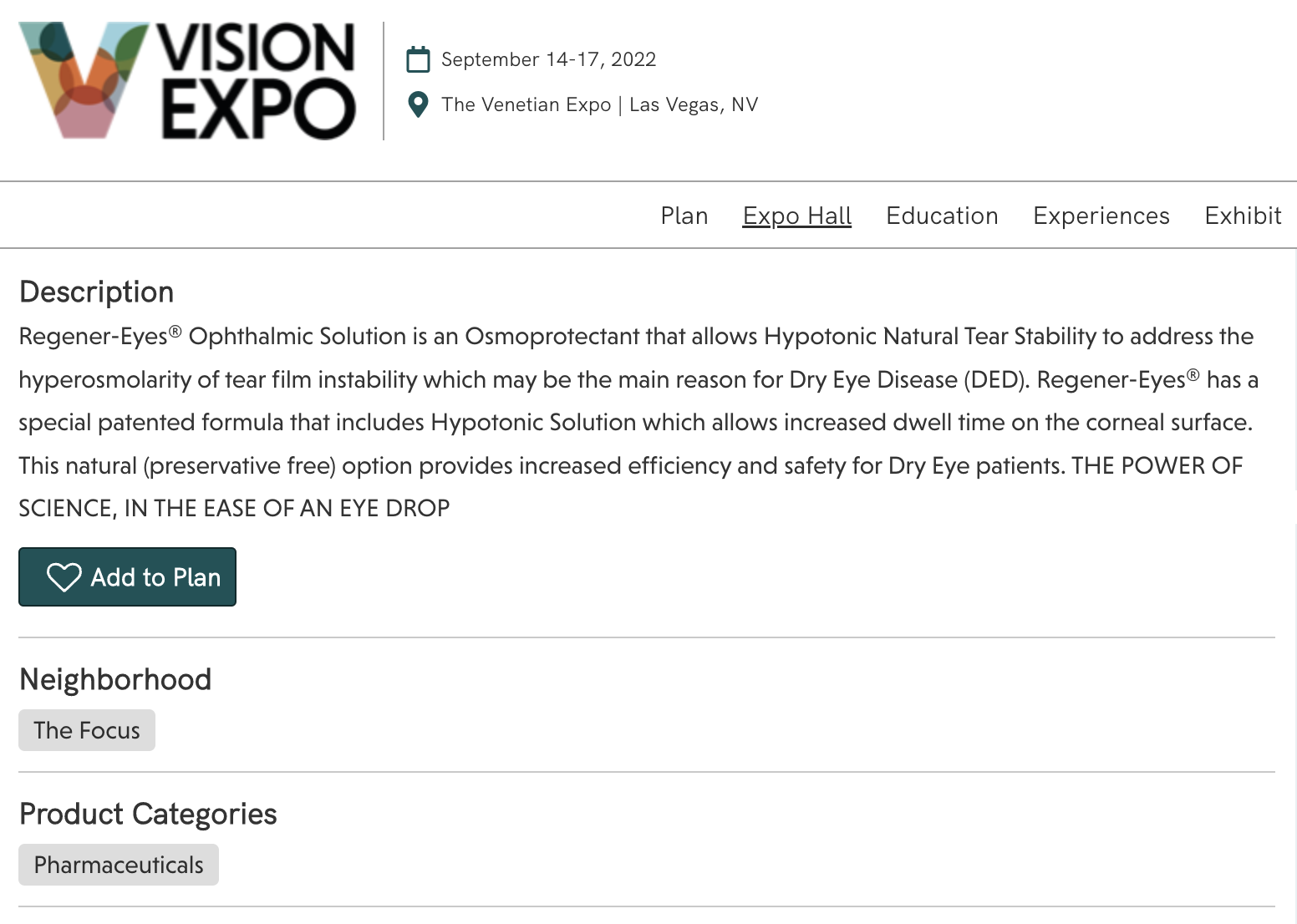
Which one is it, Regener-Eyes?
Regener-Eyes is currently spreading contradictory information at trade shows.
Here’s what they had on display the morning of September 15 at Vision Expo West. It definitely caught our eye.
1 - Excerpt from their new brochure:
2 - Extract from the white paper available at their booth, “Therapeutic potential of Regener-Eyes® Ophthalmic Solution in the treatment of dry eye disease”
Conclusion
Regener-Eyes® drops are a topical therapy for DED; they are an engineered biological product. The drops contain a large number of anti-inflammatory and trophic factors that attenuate the detrimental immune response in the eye and protect the epithelial cells of the ocular surface from injury and inflammation.1,9,12–13,15 Topical administration of Regener-Eyes® may suppress ongoing ocular inflammation, may improve meibomian gland function, and may enhance the restoration of the ocular surface barrier in DED patients, without causing treatment-related adverse events.1,13 Due to its potent immunosuppressive and regenerative properties, Regener-Eyes® should be considered as a powerful new therapeutic option in the management of DED. (emphasis our own)
Recent changes to their site
After
Before
Confused yet?
Regener-Eyes’ listing states they’re here advertising their Hypotonic drop, yet they’re handing out the white paper saying their product is biologic.
Which one is it?
Download files: