March 9, 2023 Open Letter to M2 Biologics
Download PDF
March 9, 2023
Jordan Morgan, President
M2Biologics LLC
510 Commerce Park Drive SE
Marietta, GA 30060
OPEN LETTER TO M2 Biologics LLC
What is in StimulEyes?
Is the bottle safe?
Dear Mr. Morgan:
The Dry Eye Foundation is a 501(c)(3) nonprofit organization (1) serving the ocular surface disease and ocular surface pain patient communities. We provide platforms for mutual education, support and encouragement across the international community of patients, and we advocate for patient-centered research into effective treatments for the multi-factorial conditions collectively referred to as dry eye disease.
As part of our education mission, we are actively engaged in raising awareness about safety trends of concern in the over-the-counter eye drop space (2) (3).
In January 2022, we first began investigating safety concerns related to biologic eye drops on behalf of our community. The primary concern was the bottle types, which offer no protection against bacterial contamination, in apparent violation of the Code of Federal Regulations Title 21 Section 200.50(b) (4).
In April 2022, Dry Eye Foundation began communicating with the Food and Drug Administration’s Center for Biologics Evaluation and Research about biologic eye drops. Our stated concerns included but were not limited to: (a) absence of a backwash prevention device in the bottle, which we believed to be unprecedented for commercialized preservative-free multi-dose OTC substances intended for use on the ocular surface; (b) absence of a stated indication for use; and (c) absence of evidence that clinical trials had been completed to establish the safety of the products. CBER confirmed to us that products which contain or are derived from amniotic fluid require premarket approval in order to be marketed for any medical application (5).
In April 2022, I received a communication from M2Biologics’ Sales Director stating that StimulEyes (a) is “only available by prescription from a licensed provider”, and “is a biologic, not a drug”.(6) M2Biologics’ website stated that “StimulEyes relies on its unique matrix, which is derived safely and ethically from human amniotic fluid. Its biological makeup is hyaluronic acid, cytokines, and growth factors” and that “clinical trials are not required” (7).
In May 2022, Dry Eye Foundation launched a public education campaign about biologic eye drop safety. This campaign began with presentations by our medical advisor (8), the first of which (9) addresses concerns associated with potential bacterial contamination of preservative-free eye drops in multi-dose bottles.
In July 2022, Dry Eye Foundation publicly identified StimulEyes as a biologic eye drop of concern (10). In ensuing months our medical advisor produced several educational presentations related to StimulEyes.
On September 20, 2022, the Food and Drug Administration made public a Warning Letter to Biolab Sciences, the former contract manufacturer of StimulEyes (11).
By September 28, 2022, M2Biologics had removed all content from its public-facing website except the home page, which stated: “A Regenerative Medicine Solution For Dry Eyes / StimulEyes relieves the symptoms of dry eye by reducing inflammation and providing lubrication” (12).
On November 5, 2022, attorneys for Neobiosis, the current contract manufacturer of StimulEyes, sent a Demand to Cease and Desist letter to Dry Eye Foundation and its medical advisor.
On November 17, 2022, the Food and Drug Administration sent an Untitled Letter to M2Biologics (13).
By December 11, 2022, M2Biologics had launched a new site, with a portal for consumers and eye care providers to purchase StimulEyes. The new site contains no language referencing biomaterials. (14).
On February 1, 2023, the Center for Disease Control and Prevention issued an emergency HAN alert (15) about Ezricare Artificial Tears, which has been linked to a multi-state outbreak of multi-drug resistant Pseudomonas aeruginosa infections, with outcomes including loss of vision and one death. Ezricare Artificial Tears and Delsam Pharma Artificial Tears have since been recalled (16). Both CDC and FDA drew attention to the fact that these products, though preservative-free, were packaged in [simple] multi-dose bottles (15) (17).
On February 21 2023, I purchased StimulEyes online ($229) from M2Biologics’ website, which says it “contains no preservatives” and describes it as having “natural, scientifically studied ingredients including hyaluronic acid” (Image 1). My order was later refunded. There is a notice on the site stating that online ordering is currently unavailable (Image 2).
On February 25, 2023, I purchased StimulEyes from dryeyerescue.com.
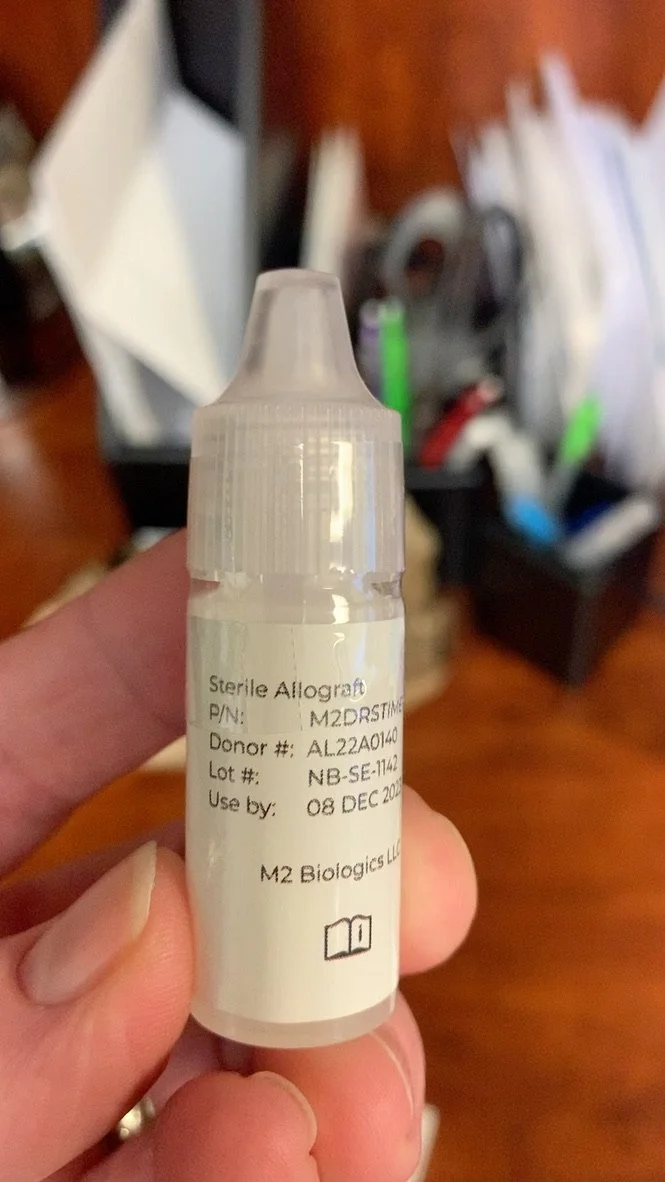
On Monday, March 6, I received my shipment. The bottle is of a conventional type with no MDPF dropper to prevent contamination of the contents after opening (18). The label calls it an “allograft” (Image 3). The package insert mentions “bio-active components” and “donor biomaterial” (Image 4). None of this information was on either M2Biologics’ website (Images 1, 2) or DryEyeRescue’s website (Image 5).
We therefore ask in this Open Letter:
1. What is in StimulEyes? Is it biologic? Is it preservative-free, as advertised?
StimulEyes has no National Drug Code. It is not listed on DailyMed.
There is no ingredient list on the StimulEyes bottle, box or package insert.
2. If StimulEyes is preservative-free, how is bacterial contamination prevented during use, given that it is packaged in a simple multi-dose bottle?
The dropper visibly sucks liquid back into the bottle after dispensing a drop. (18)
The package insert states “Product can be stored at ambient temperature… up to the ‘Use by’ date”.(Image 5)
What evidence do you have that this packaging protects the contents from bacterial contamination in satisfaction of 21 CFR 200.50(b)?
The Dry Eye Foundation eagerly anticipates a definitive resolution to the questions of what is in StimulEyes and whether it is subject to bacterial contamination after opening.
Sincerely,
Rebecca Petris
President
NOTES
http://www.dryeyefoundation.org
https://www.ecfr.gov/current/title-21/chapter-I/subchapter-C/part-200/subpart-C/section-200.50
Email communication, April 6, 2022
Email communication, April 27, 2022
https://web.archive.org/web/20220704173939/https://www.m2biologics.com/stimuleyes/faq/
Sandra Brown MD, Board of Directors, Dry Eye Foundation
https://www.biologiceyedrops.org/non-preserved-eye-drop-safety
https://www.biologiceyedrops.org/watch/fda-approval-for-biologic-eye-drops
https://web.archive.org/web/20220928053241/https://www.m2biologics.com/
https://web.archive.org/web/20221211152211/https://www.m2biologics.com/
COPIES TO:
Food and Drug Administration
Center for Biologics Evaluation and Research
Melissa Mendoza, JD - Food and Drug Administration, Center for Biologics Evaluation and Research, Office of Compliance and Biologics Quality
Robert Sausville, Director, Division of Case Management
Maria Anderson, Supervisory Consumer Safety Officer, Biological Drug and Device Compliance Branch
Monique Lester, Consumer Safety Officer, Biological Drug and Device Compliance Branch
Jonathan Swoboda, Consumer Safety Officer, Biological Drug and Device Compliance Branch
Center for Drug Evaluation and Research
Carolyn Becker, JD, Office of Unapproved Drugs and Labeling Compliance
Image 1
This screenshot represents the entirety of the “About StimulEyes” section of https://www.m2biologics.com/ as of 3/9/23.
Image 2
https://www.m2biologics.com/ as of 3/9/2023
Image 3
Image 4
(Outside)